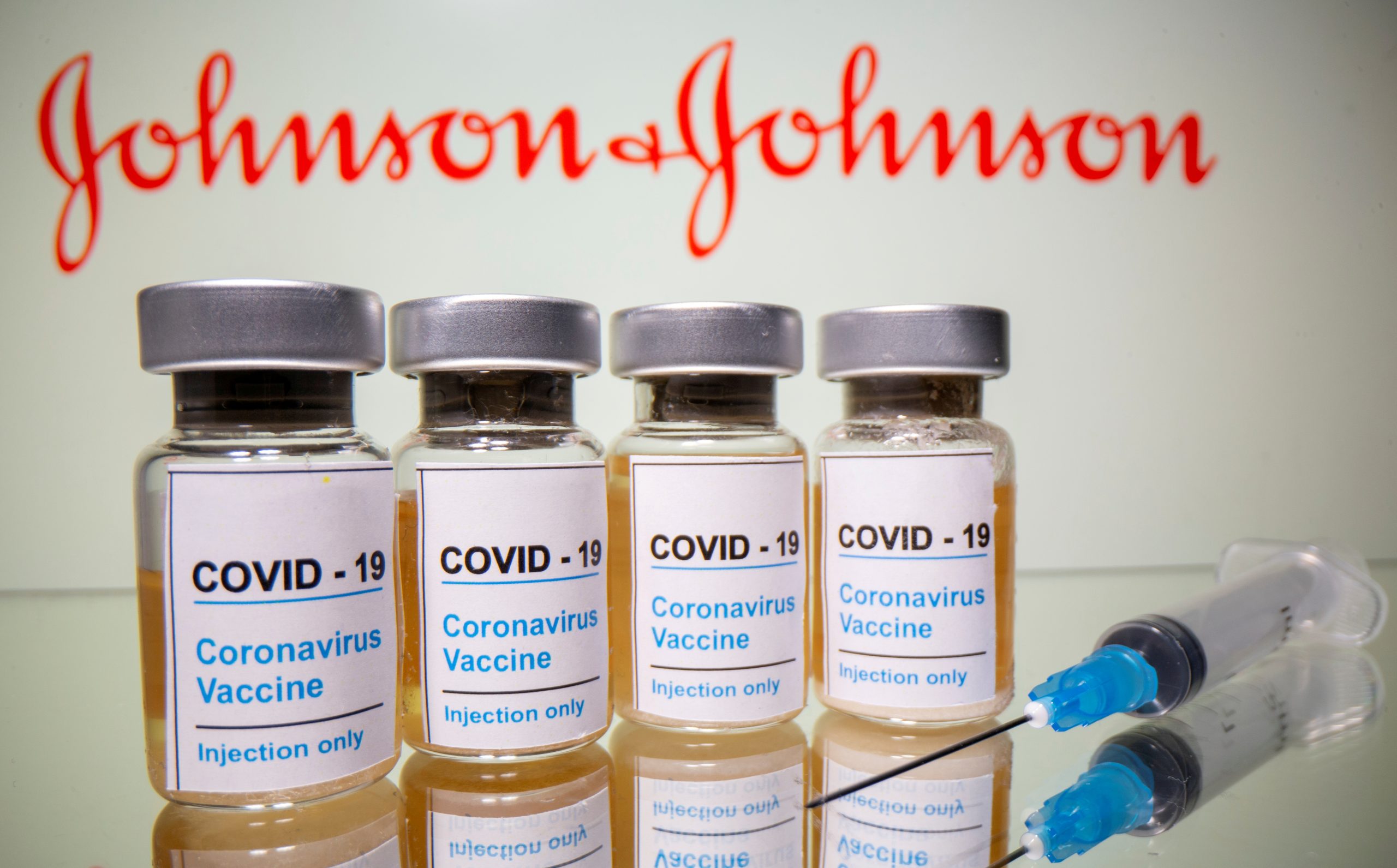
A U.S. health panel on Wednesday agreed they wanted more data before voting to resume vaccinations with Johnson & Johnson’s COVID-19 shot, even as a U.S. Food and Drug Administration scientist said warnings could mitigate the risk of rare but serious blood clots.
The advisory panel aims to reconvene in a week to 10 days.
The panel is reviewing six reported cases of rare brain blood clots in women who received the J&J vaccine, a day after federal regulators paused its use to assess the issue.
Dr. Lynn Batha, an epidemiologist at the Minnesota health department, and several others spoke in favor of extending the pause to gather more safety information.
“By having more robust information, I think we can be more confident about how we talk about the safety of this vaccine,” she told other members of the U.S. Centers for Disease Control and Prevention (CDC) advisory panel.
Earlier, the FDA’s deputy director for vaccine development, Doran Fink, told the CDC panel that his current thinking was that warning statements and communications from the federal agency would allow doctors to weigh risks and benefits of the vaccine.
Other panel members, however, expressed concern that extending the pause could worsen issues related to equitable access to the vaccine, which is seen as important for serving hard to reach communities because it can be stored in normal refrigerator temperatures and is given as one dose instead of two.
(Reporting by Susan Heavey, Mike Erman, Julie Steenhuysen and Manas Mishra; Editing by Peter Henderson and Bill Berkrot)





















 Continue with Google
Continue with Google