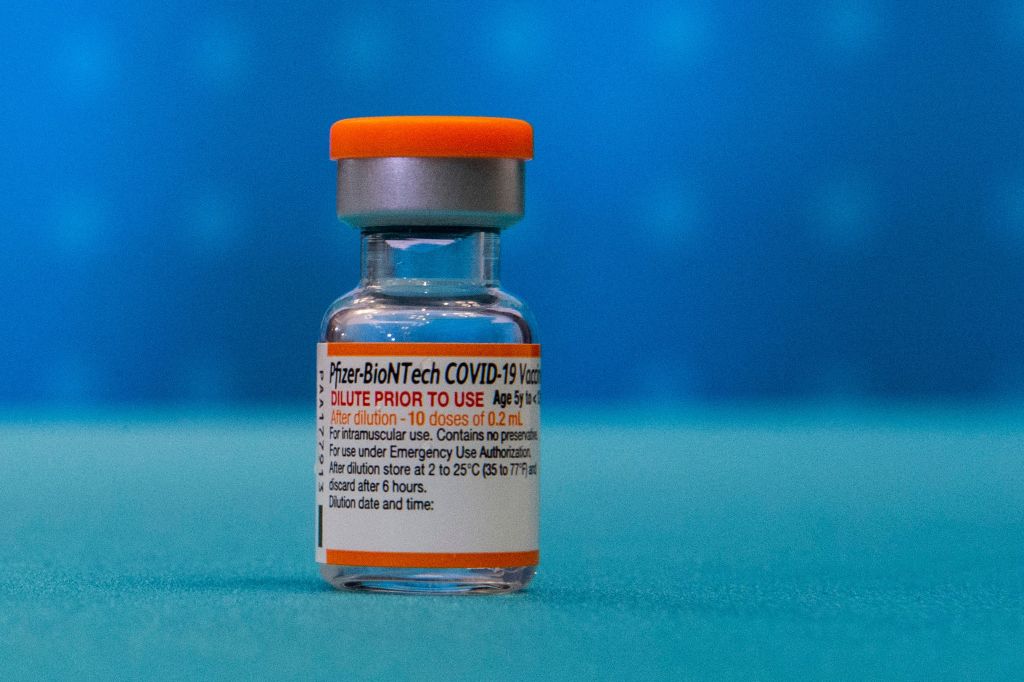
A Centers for Disease Control and Prevention advisory committee on Friday called for a greater gap between doses of the Pfizer-BioNTech and Moderna vaccines.
The CDC’s Advisory Committee on Immunization Practices backed changing the time between doses to eight weeks, according to NBC News.
The panel said the change would improve the effectiveness of the vaccines in providing protection against the coronavirus and should lower the risk of a rare side effect as well.
Currently, three weeks separate the first two doses of the Pfizer vaccine. The time span is four weeks for Moderna. The original time span was determined at a time when the prime concern was getting as many people vaccinated as fast as possible.
A presentation given to the panel by Dr. Sara Oliver, a CDC epidemic intelligence service officer, said the longer interval is expected to reduce the chances of vaccine recipients getting myocarditis, an inflammation of the heart.
According to NBC News, Oliver noted that the Pfizer and Moderna vaccines both appear linked to elevated but still rare cases of myocarditis, with the Moderna vaccine showing a stronger link. She said in her presentation that the rates for this side effect peak after the second dose and appear to target younger men more than anyone else.
The presentation said that a longer gap between doses also could improve the effectiveness of the vaccines.
According to Oliver, about 33 million Americans between the ages of 12 and 39 who have not yet been vaccinated could be impacted by the change
Dr. Grace Lee, who chairs the committee, said the panel will seek to have the recommendation included in agency guidance.
Myocarditis risks caused some European countries to put Moderna’s vaccine on pause. Dr. Bryna Warshawsky, a medical adviser with the Public Health Agency of Canada, said research has shown myocarditis rates fell when the gap between doses was lengthened, according to NBC News.
The lowest rates came with an eight-week gap, Warshawsky said.
Data supplied to the CDC by Canadian researchers said the eight-week gap also showed higher antibody responses and higher vaccine effectiveness, the report said.
Panel members supported the change.
“As we approach these new variants, the higher antibodies and the more diverse these antibodies are, the better protected people will be,” said Dr. Helen Keipp Talbot, a committee member and a professor of medicine at Vanderbilt University. “I think it’s a win-win for both safety and immunogenicity spread out.”
Dr. Matthew Daley, a senior investigator at Kaiser Permanente Colorado and member of the panel, said the change can address safety concerns.
“Vaccine safety is raised again and again,” Daley said, according to NBC News. “We have vaccines that are highly effective and have a high degree of safety, but we have a way to make them even safer.”
Bill Hanage, a Harvard epidemiologist not on the panel, said changing the dosage schedule in a massive project with a brand new drug is not a big deal.
“Changes to dosing schedules happen all the time,” he said. “They don’t mean other schedules were necessarily bad, but that we can’t test all schedules at one time and so we refine them as data accumulate.”
This article appeared originally on The Western Journal.





















 Continue with Google
Continue with Google