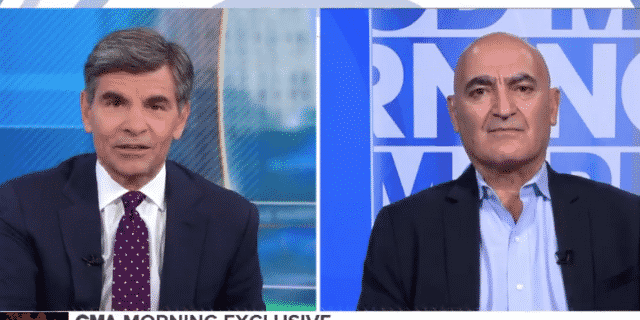
“Operation Warp Speed” Chief Science Adviser Dr. Mocef Slaoui is being pressed for clarity on President Donald Trump’s forthcoming executive order.
Trump is planning to sign an executive order on Tuesday to order priority access of COVID-19 vaccines obtained by the U.S. government to Americans before helping other countries.
Slaoui was pressed by ABC News’ George Stephanopoulos during Tuesday’s interview for clarity regarding the executive order.
When asking for an explanation on the executive order, Stephanopoulos said, “I don’t quite understand it. [Trump’s] saying that foreign countries aren’t going to be able to get the vaccine until everybody here in the United States gets it. It sounds like the problem is the opposite right now.”
“Pfizer has made deals with other countries that are going to limit the supply here,” the ABC News host noted.
Slaouci responded, “Frankly I don’t know, and frankly, I’m staying out of this. I can’t comment.”
“You don’t know?” Stephanopoulos shot back, adding, “You’re the chief science adviser for ‘Operation Warp Speed.'”
Slaouci sought to defend himself, saying, “Our work is rolling. We have plans. We feel that we can deliver the vaccines as needed, so I don’t know exactly what this order is all about.”
Watch the interview below:
BREAKING: Pressed by @GStephanopoulos to explain Pres. Trump's executive order prioritizing Americans’ access to COVID-19 vaccines before the United States helps other countries, "Operation Warp Speed" Chief Science Adviser Dr. Moncef Slaoui says, "Frankly I don't know." pic.twitter.com/Wk7ElJKDaw
— Good Morning America (@GMA) December 8, 2020
Earlier in the interview, Stephanopoulos noted Pfizer may not have the doses needed in the U.S. until June or July of 2021 due to supply chain issues.
Slaouci, however, said that the U.S. is confident that there will be COVID-19 vaccines available to those who need them “as soon as possible.” He added that they would work with Pfizer to “try to increase capacity and have those vaccines available.” He also noted that there are other companies creating potential COVID-19 vaccines.
The “Operation Warp Speed” chief science adviser reiterated the promise to “immunize the U.S. population” by mid-year of 2021, which he says is still on track.
Pfizer, which worked with BioNTech, and Moderna have created COVID-19 vaccine candidates. They have applied for U.S. Food and Drug Administration (FDA) authorization on their candidate vaccines. The FDA will hold an emergency use authorization hearing for Pfizer on Thursday and for Moderna on Dec. 17, according to Fox Business.
The Trump administration reportedly turned down buying additional doses of the Pfizer vaccine candidate in late summer, according to The New York Times. This creates “concerns that Pfizer would be unable to fulfill any additional U.S. order until June because of the company’s commitments to other countries,” CNN reported.
Britain began mass vaccination for the coronavirus for the most vulnerable — the first Western nation to do so. It is also the first globally to rollout the Pfizer shot.
Trump plans to speak at the Operation Warp Speed Vaccine Summit at the White House on Tuesday.
























 Continue with Google
Continue with Google