House Speaker Nancy Pelosi (D-Calif) is weighing in with deep concern about President Donald Trump’s health following his decision to take an unproven drug to treat the coronavirus, hydroxychloroquine.
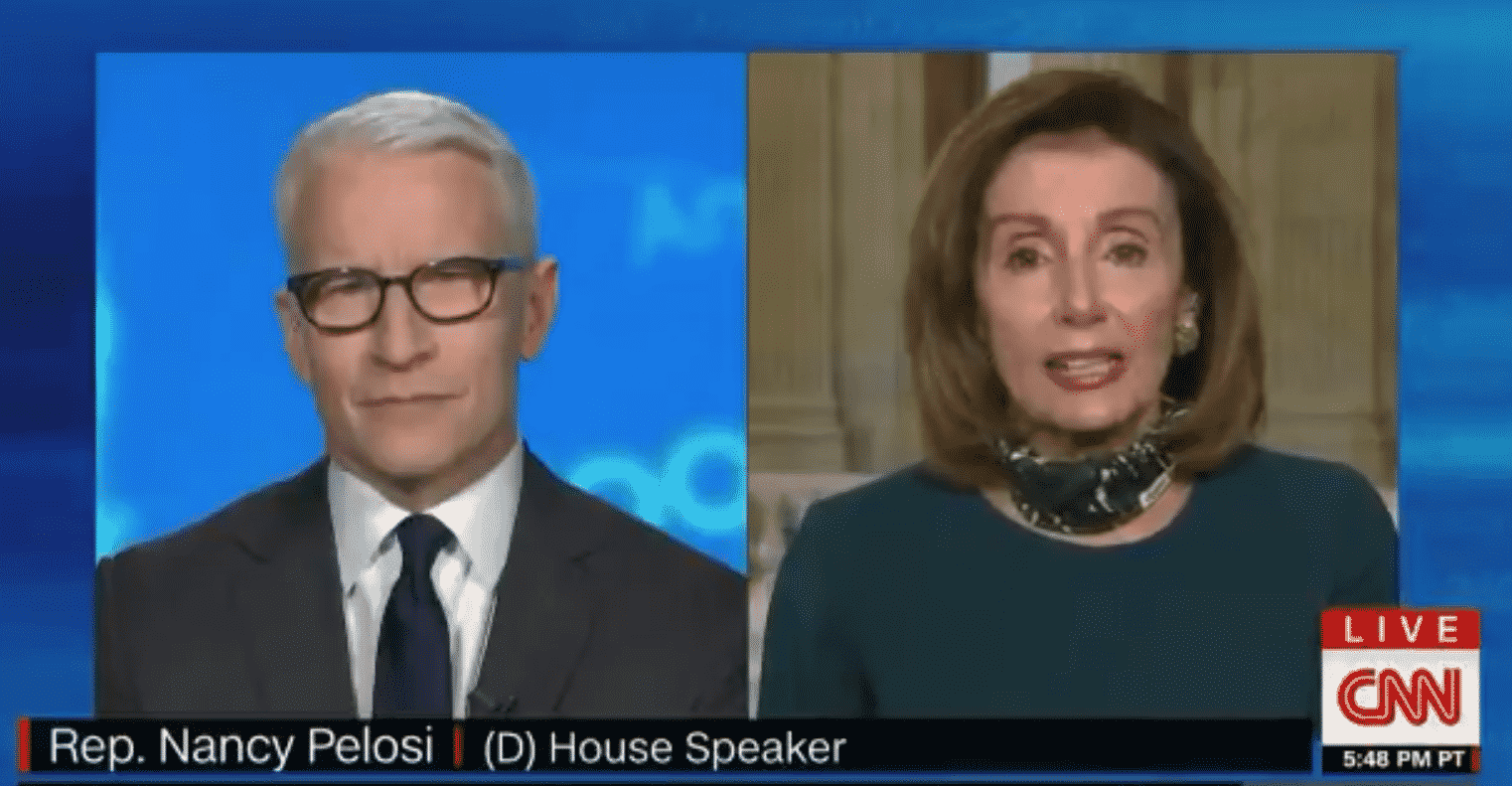
Pelosi appeared on CNN’s “Anderson Cooper 360” on Monday evening where she was asked about the president’s admission to taking the drug as a preventative measure for the coronavirus.
Describing the president as “morbidly obese” while noting brief details about the president’s health, the Democratic lawmaker admitted she would rather the president not take the drug.
“He’s our president and I would rather he not be taking something that has not been approved by the scientists, especially in his age group and in his, shall we say, weight group, what is morbidly obese, they say,” Pelosi said.
“So, I think that it’s not a good idea,” she said.
See Pelosi’s remarks below:
“I would rather he not be taking something that has not been approved by the scientists, especially in his age group, and in his, shall we say, weight group: ‘Morbidly obese,’ they say,” says House Speaker Nancy Pelosi on Pres. Trump’s revelation he is taking hydroxychloroquine. pic.twitter.com/0ImjpEjg9q
— Anderson Cooper 360° (@AC360) May 19, 2020
Pelosi’s interview came just hours after Trump turned heads during the live round-table discussion on Monday where he admitted that he had been taking hydroxychloroquine for more than a week, as IJR reported.
“I’m taking hydroxychloroquine,” Trump said, adding, “I’ve been taking it for the last week and a half. A pill every day.”
He also claimed frontline workers have been taking the drug as a preventative drug.
Trump says he’s been taking hydroxychloroquine to ward off #coronavirus, despite lack of evidence about its effectiveness. pic.twitter.com/SgjpAJfLPe
— Al Jazeera English (@AJEnglish) May 19, 2020
Since March, Trump has touted the drug and at one point he even described it as a potential “game-changer” in the battle against the coronavirus. However, studies have suggested there is no evidence the anti-malarial drug is sufficient to treat the coronavirus.
Although the president has argued that the drug has been around for decades, the National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and Food and Drug Administration (FDA) have all denounced the drug in some capacity or issued some form of warning advising against using the drug as a form of treatment for COVID-19.
Despite the warnings, the White House released a brief letter from presidential physician Sean Conley. He admitted that he and the president discussed the drug and ultimately determined “the potential benefit from treatment outweighed the relative risks.”
Conley wrote, “In consultation with our inter-agency partners and subject matter experts around the country, I continue to monitor the myriad studies investigating potential COVID-19 therapies, and I anticipate employing the same shared medical decision making based on the evidence at hand in the future.”
























 Continue with Google
Continue with Google